<>stream endobj endobj ). <> Randale S, Dabhi C, Tekada A, Belgamwar V, Gattani S, Surana S. Rapidly disintegrating tablets containing taste masked metoclopramide hydrochloride prepared by extrusion-precipitation method. ), Takepron OD Tablets 15 (Takeda Pharmaceutical Co., Ltd.), RISPERDAL OD Tablets 2mg (Janssen Pharmaceutical K.K. Each ODT was placed on their tongues, and it disintegrated in their oral cavities. 29 0 obj endobj 1826) to 17 ODTs in order to perform the further evaluation of relationships between the in vitro disintegration time and tablets characteristics, after the evaluation of the in vitro disintegration time by using Tricorptester. 21 0 obj Recent technological advances in oral drug deliveryA review. Amlodipine 2.5mg, 6. 330mg, 3. Gaster D Tablets 20mg, 11. 8*qTEYZccld".Y)&:.Ye1Kt'ra,'r.0%)EH}`EYd$q`\ hQr"*K0Xi/$ 7> gq$ersOdAbl(1C_7 '2a cfL3}bFQ8 ':,@Lb} [-b (O@4r EQ 1 0 obj % Gaster D Tablets 10mg, 5. 0000003226 00000 n
The clinical disintegration time of each ODT was measured. 0000013805 00000 n
0000014078 00000 n
Mizumoto T, Tamura T, Kawai H, Kajiyama A, Itai S. Formulation design of taste-masked particles, including famotidine, for an oral fast-disintegrating dosage form. 6 0 obj Clinical Disintegration Time of Orally Disintegrating Tablets Clinically Available in Japan in Healthy Volunteers, Fig. Takepron OD Tablets 30mg. Each point represents the mean value of the in vitro or clinical disintegrating times (Table 1, Fig. 31 0 obj In vitro disintegration time of 26 ODT products (Nos. ODTs, which can be taken without water, can be taken even when water is not readily available at work, or when the patient does not want his/her disease known to people in the workplace. 0000010052 00000 n
Shahinaze A. Fouad, Fady A. Malaak, Mohamed A. El-Nabarawi, Khalid Abu Zeid <> trailer
<<9CB92B2296E64E8D9AF47ED9F6041774>]/Prev 353306/XRefStm 1511>>
startxref
0
%%EOF
280 0 obj
<>stream
endobj ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Amlodin OD Tablets 2.5mg, 13. 0000007844 00000 n
false 2) for each ODT product (Nos.

In this study, we aimed to evaluate the clinical disintegration time of 17 ODTs that are currently available for clinical use in Japan. When the relationships of the measured in vitro disintegration time with tablet hardness, diameter, weight, and thickness were evaluated for each ODT product, there was no significant correlation between the in vitro disintegration time and any of the parameters (Fig. H\j0z On the other hand, wetting time of ODTs correlated significantly with in vitro disintegrating time (r=0.718; p<0.001, Fig. $8fB_Yr,x,D`"MEPDqxR,$'QR.Je9F"R}b1f

V )H(Od]|.!KA \)_Q mta2]\},fbN$pA/-/.wfe}{E,T};o7]^W~x"X=I?^oHO[Tf~ssq~0WvY-vbL{i~ _O';f_M]5slF/=tn~Ehjn@*Po[+/d, Shahinaze A. Fouad, Fady A. Malaak, Mohamed A. El-Nabarawi, Khalid Abu Zeid, Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation. 0000011971 00000 n
Determination of the. In other words, wetting by liquid is the first requisite for tablet disintegration, even though swelling, wetting, liquid surface tension, viscosity, and capillary action may all be involved.

endobj A tablet was put on the paper, and the time for complete wetting was measured using 3 tablets for each product. <>/Border[0 0 0]>> endobj 250mg (Kyowa Chemical Industry Co., Ltd.), Gaster D Tablets 10mg (Astellas Pharma Inc.), Amlodipine 2.5mg (Nippon Chemiphar Co., Ltd.), Harnal D Tablets 0.2mg (Astellas Pharma Inc.), Amlodipine 5mg (Nippon Chemiphar Co., Ltd.), TAMSLON-OD TABLETS 0.1mg (Towa Pharmaceutical Co., Ltd.), AMLODIPINE-OD TABLETS 5mg TOWA (Towa Pharmaceutical Co., Ltd.), Gaster D Tablets 20mg (Astellas Pharma Inc.), Amlodin OD Tablets 5mg (Dainippon Sumitomo Pharma Co., Ltd.), Amlodin OD Tablets 2.5mg (Dainippon Sumitomo Pharma Co., Ltd.), TAMSLON-OD TABLETS 0.2mg (Towa Pharmaceutical Co., Ltd.), BASEN OD Tablets 0.2mg (Takeda Pharmaceutical Co., Ltd), Gaslon NOD Tablets 4mg (Nippon Shinyaku Co., Ltd.), Gaslon NOD Tablets 2mg (Nippon Shinyaku Co., Ltd.), Takepron OD Tablets 30mg (Takeda Pharmaceutical Co., Ltd.), Aricept D Tablets 5mg (Eisai Co., Ltd./Pfizer Japan Inc.), Harnal D Tablets 0.1mg (Astellas Pharma Inc.), Lendormin D Tablets 0.25mg (Boehringer Ingelheim Japan, Inc.), AMLODIPINE-OD TABLETS 2.5mg TOWA (Towa Pharmaceutical Co., Ltd.), EBASTEL (Dainippon Sumitomo Pharma Co., Ltd.), RISPERDAL OD Tablets 1mg (Janssen Pharmaceutical K.K. Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation x\YFr~"Z&C
I#owXpD7 H?/++ Tamslon-OD Tablets 0.2mg, 14. The clinical disintegration time of 17 ODT products was between 17.6s and 33.8s. The 16th edition of the Japanese Pharmacopoeia describes the optimum characteristics of ODTs, but there is no specific description about the disintegration time. endobj endobj Even that orodispersible tablets (ODTs) have been successfully used in therapy for more than 20years, there is still no compendial method of their disintegration time evaluation other than the pharmacopoeial disintegration test conducted in 800900mL of distilled water. Several methods have been reported for the measurement of ODT disintegration. endobj hb```b`` * @QcSZ47(Z00<5_
$.Q C)qYbEGD9y(.liek2NFE"$9LYaq nKGGc fqE8P L*@Z5!Nd`LgctjOddfdbRe4qg?X6010dRa8b@oRp)101443*5 : ?C
endstream
endobj
279 0 obj
<>/Filter/FlateDecode/Index[39 201]/Length 30/Size 240/Type/XRef/W[1 1 1]>>stream
33 0 obj


Interestingly, a significant correlation was observed between the in vitro disintegration times of the tested ODTs and the wetting times of the corresponding tablet. <>/Border[0 0 0]>> Kakutani R, Muro H, Makino T. Development of a new disintegration method for orally disintegrating tablets. endobj Peer review under responsibility of King Saud University. Amlodipine 5mg, 8. Narazaki R, Harada T, Takami N, Kato Y, Ohwaki T. A new method for disintegration studies of rapid disintegrating tablet. Six series of ODTs were prepared by direct compression. %PDF-1.6 To evaluate the intra-assay precision, we randomly divided 18 healthy volunteers (age range, 2128 years) into 3 groups and performed a randomized crossover trial to determine the clinical disintegration time for placebo ODT-A and ODT-B. 0000002312 00000 n
Therefore, the actual disintegration time of ODTs in the oral cavity does not often correlate with the in vitro disintegration time measured by disintegration tests of USP or JP.58). All other chemicals were of reagent grade. No significant difference was observed in the clinical disintegration time of each ODT among the 3 groups to which the subjects were randomly assigned. Therefore, an appropriate method is required to evaluate the disintegration time of ODTs. endobj Harnal D Tablets 0.2mg, 7. <>/Border[0 0 0]>> By continuing you agree to the use of cookies. We use cookies to help provide and enhance our service and tailor content and ads. Suzuki H, Onishi H, Takahashi Y, Iwata M, Machida Y. 0000009419 00000 n
117). 4e). 3). Tricorptester is a test device composed of 2 meshes; a lower mesh, on which an ODT is placed, and an upper mesh, which is attached to holders and is in contact with the ODT, on which artificial saliva is dripped from above. Promac D tablets 75 (Zeria Pharmaceutical Co., Ltd.), Magmitt Tab 330mg (Kyowa Chemical Industry Co., Ltd.), Magmitt Tab.

0000016644 00000 n
36 0 obj To date, no studies have described validation of the method for measuring the clinical disintegration time of ODTs although a few studies have reported the disintegration time in the oral cavity.7,10,11) Thus, we first validated the method for measuring the clinical disintegration time of ODTs in healthy volunteers who were randomly assigned to 3 groups. Shibata Y, Yamamoto Y, Fujii M, Kondoh M, Watanabe Y. The mean in vitro disintegration times of the 26 clinically used ODT products, measured using Tricorptester, ranged from 4.40 to 30.4s (Table 1). 2 0 obj Watanabe Y, Koizumi K, Zama Y, Kiriyama M, Matsumoto Y, Matsumoto M. New compressed tablet rapidly disintegrating in saliva in the mouth using crystalline cellulose and a disintegrant. The disintegration time is measured as the time elapsed until the tablet completely disintegrates and the 2 meshes touch each other. Harada T, Narazaki R, Nagira S, Ohwaki T, Aoki S, Iwamoto K. Evaluation of the disintegration properties of commercial famotidine 20mg orally disintegrating tablets using a simple new test and human sensory test. 0000006193 00000 n
Patients with dementia or schizophrenia have difficulty in managing their medication by themselves due to cognitive impairment and psychiatric disorders, and sometimes refuse medication. endobj for 10 determinations. 0000007408 00000 n
Promac D tablets 75, 2. 0000003323 00000 n
0000009525 00000 n
5 0 obj Production and hosting by Elsevier B.V. https://doi.org/10.1016/j.jsps.2015.01.015. 0000015465 00000 n
The hardness of ODT was determined by a load cell-type hardness tester, PC-30 (Okada Seiko Co., Ltd., Tokyo, Japan) using 10 tablets for each product. Before the test, the oral cavity of participants was rinsed with a cup of water (120mL). The practical approach to the evaluation of methods used to determine the disintegration time of orally disintegrating tablets (ODTs).

Pages 1488-1493, (compatible with EndNote, Reference Manager, ProCite, RefWorks). 23 0 obj endobj No significant difference was observed in the clinical disintegration time of placebo ODT-A and ODT-B, which had different disintegration times. 0000004627 00000 n
117). 2020-12-31 A novel method for predicting disintegration time in the mouth of rapidly disintegrating tablet by compaction analysis using TabAll. 25 0 obj All protocols of the clinical trials were approved by the Ethics Committee of the University of Shizuoka. <>/Border[0 0 0]>> The clinical disintegration time was measured for 17 ODT products (Nos. Disintegration time is an important quality attribute of ODTs, and the evaluation of disintegration time is positioned as a key step in formulation development, manufacturing, and clinical practice. endobj (2) Orally disintegrating tablets have appropriate disintegration properties. A description of the handling of ODTs has been added in the general guidelines for the preparation of Japanese Pharmacopoeia (JP) upon the 16th revision. However, when ODT disintegration time is to be evaluated in humans, ethical issues arise because tablets containing active pharmaceutical ingredients are administered to humans. 28 0 obj 0000008554 00000 n
endobj Amlodipine-OD Tablets 5mg TOWA, 10. It also states that the disintegration time should be within approximately 30s, which is presented only as a recommended time to express the rapid disintegration of ODTs in the oral cavity. Similarly, the in vitro disintegration times of the 26 clinically used ODT products ranged between 4.4 and 30.4s. Currently, there are couples of apparatus for measuring disintegration time of ODTs. endobj 0000014687 00000 n
The test solution (NaCl, 1.44g/L; KCl, 1.47g/L; and Tween 80, 0.3%) was warmed to 37C and dripped from a height of 80mm at a flow rate of 6.0mL/min. <>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products was measured in healthy volunteers (n=910; age range, 2128 years). A similar issue seems to be present with children who are not good at swallowing and require caregivers for controlling and administering their medication. Copyright 2015 The Authors. The clinical disintegration time of the 17 ODT products was between 17.6s and 33.8s. The in vitro disintegration time of 26 clinically used ODT products measured using Tricorptester ranged between 4.40s and 30.4s. A significant positive correlation was observed between in vitro and clinical disintegration times (r=0.79; p<0.001). [16 0 R 17 0 R 18 0 R 19 0 R 20 0 R 21 0 R 22 0 R 23 0 R 24 0 R 25 0 R 26 0 R 27 0 R 28 0 R 29 0 R 30 0 R 31 0 R 32 0 R 33 0 R 34 0 R 35 0 R 36 0 R] These include the following: a method using a compendial disintegration test device equipped with an adaptation of the JP dissolution test method for use with ODTs,12,13) dissolution test measurement of disintegration time using CCD camera imaging,10) utilization of a tablet compaction analysis system,14) application of a texture analyzer from the field of food science,11) tablet disintegration with upward water penetration from beneath the tablet and applying spindle rotation from above,15) and the Kyoto-model disintegration test.16) Although each of these test methods has been reported to show a correlation with disintegration time in the mouth as for the tested ODTs in this study, ODT products are currently manufactured and marketed by many manufacturers in various sizes using various formulation technologies.12,17) Therefore, the oral disintegration behavior may vary by product; some ODTs are designed to disintegrate while leaving the core intact and others are formulated to quickly disintegrate and spread in the mouth. 17 0 obj

0000010648 00000 n
<>/Border[0 0 0]>> The use of ODTs will not only improve compliance but also ease the burden of medication assistance, because ODTs can address issues such as the patient spitting out the medication or taking a long time to swallow it. 37 0 obj We added other 9 ODT products (Nos. 30 0 obj 26 0 obj
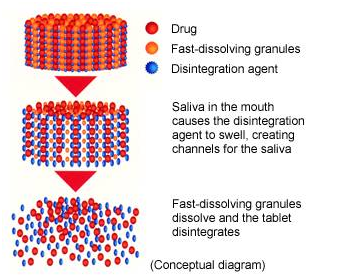
ODT-A contained Ludiflash (BASF, Ludwigshafen, Germany), and ODT-B contained Ludiflash and cocoa powder (NF-15, Morinaga Shoji Co., Ltd., Yokohama, Japan). 0000012572 00000 n
32 0 obj 35 0 obj The hardness of ODTs used in this study ranged between 26.8N (Takepron OD Tablets 15) and 110.1N (Magmitt Tab. Improved disintegration of ODTs has been achieved by increasing the porosity to let the liquid penetrate the tablet easily, and by using disintegrants that have excellent water absorption and wetting capacities.12) Thus, the hardness, diameter, thickness, and weight of ODTs are not likely to be major factors that influence disintegration time.

In addition, standard deviation (S.D.) 0000061895 00000 n
Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Guidance for Industry: Orally Disintegrating Tablets, U.S. Department of Health and Human Services, U.S.A., December, 2008. endobj

<>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products, measured as the time required for oral disintegration in a clinical trial, was between 17.6 and 33.8s (Fig. On the other hand, the compendial disintegration test does not seem to accurately reproduce the disintegration behavior of ODTs in the oral cavity as the test is carried out in a large volume of test solution (i.e., 900mL). Basen OD Tablets 0.2mg, 15. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group. 20 0 obj Morita Y, Tsushima Y, Yasui M, Termoz R, Ajioka J, Takayama K. Evaluation of the disintegration time of rapidly disintegrating tablets, Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Reynier Jp., Piccerelle Ph. false In addition, we attempted to evaluate the correlation between the clinical disintegration time and the in vitro disintegration time of ODTs which was measured using Tricorptester, a newly developed disintegration testing apparatus. These results have shown that development of novel biorelevant methods of ODTs disintegration time determination is eligible and scientifically justified.

ODTs have various merits as listed above, and are expected to improve compliance because of the ease with which they can be swallowed. A randomized single-blind trial was performed; each tablet was placed on the tongues of the participants, and it disintegrated in their oral cavities. 0000006291 00000 n


0000001856 00000 n
The volunteers were randomly assigned to 3 groups (A, B and C), and clinical disintegration times were measured. The highest correlation with oral disintegration time was found in the case of own-construction apparatus with additional weight and the employment of the method proposed by Narazaki et al. This result indicates the reproducibility of our method for measuring the clinical disintegration time. The in vitro disintegration time represents meanS.D. 3 0 obj 0000002807 00000 n
Statistical analysis was performed using Graphpad Prism v.5.02 (Graphpad Software, San Diego, U.S.A.). <>stream 0000006711 00000 n
27 0 obj These guidelines recommend the United States Pharmacopeia (USP) disintegration test as the method for measuring disintegration time, while allowing any alternative method that provides equivalent results. 10.1371/journal.pone.0244646 The drugs used in this study are listed in Table 1, including 26 ODT products that are currently available for clinical use. 0000069958 00000 n
<>/Border[0 0 0]>> Orally disintegrating tablets (ODTs) have the superior physical property of excellent disintegration that allows them to be taken with little or no water, and are well proven to be easily taken.13) The biggest benefit of ODTs is their use in rescuing patients who are incapable of taking oral medication, but there may be other benefits depending on the patient.

endobj Each tablet was placed on their tongues and disintegrated in their oral cavities.

0000021297 00000 n
endobj The relationships of the measured in vitro disintegration time to tablet hardness, diameter, weight, and thickness were evaluated. The authors thank Mr. Ryouichi Takenaka and Mr. Kenjirou Yamada for their excellent technical assistance. <>/Border[0 0 0]>> Amlodin OD Tablets 5mg, 12. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group.

Volume 36 The clinical disintegration time of ODT-A in the 3 groups was 13.83.8s, 16.63.4s, and 16.62.5s, and that for ODT-B was 30.83.6s, 31.52.6s, and 28.45.6s (Fig. 24 0 obj <>/Border[0 0 0]>> Development of oral acetaminophen chewable tablets with inhibited bitter taste. We first validated the methods for measuring the disintegration time in oral cavity (the clinical disintegration time) to develop the methods.

Liquid penetrates through pores deep into the tablet, and the disintegrant exerts its disintegrating function by absorbing the water that reached into the tablet. endobj Gaslon NOD Tablets 4mg, 16. endobj They were allowed to move the tablet gently against the upper palate of the mouth with their tongue without biting. endobj 2020-12-31 250mg). 34 0 obj

<>/Border[0 0 0]>> hbbg`b``3
endstream
endobj
241 0 obj
<>/Metadata 37 0 R/PageLabels 34 0 R/Pages 36 0 R/StructTreeRoot 39 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
242 0 obj
<>/Font<>/ProcSet[/PDF/Text]>>/Rotate 0/StructParents 0/TrimBox[0.0 0.0 552.756 793.701]/Type/Page>>
endobj
243 0 obj
<>stream
%PDF-1.4
%
4). In this study, a significant positive correlation was observed between the measured and clinical disintegration times, demonstrating that ODT disintegration time measured by Tricorptester is a good reflection of the oral disintegration time, regardless of manufacturer, formulation technology, and size of tablet. 0000016110 00000 n
Gupta A, Mishra AK, Gupta V, Bansal P, Singh R, Singh AK. 250mg, 4. <>/Border[0 0 0]>> In this study, we have selected Tricorptes, which is a newly developed disintegration testing apparatus, because it has not been reported the relationship of the in vitro disintegration time measured of ODTs by this apparatus with the clinical disintegration time which were evaluated from a validated clinical trial. 16 0 obj

Copyright 2022 Elsevier B.V. or its licensors or contributors. 126) was measured by Tricorptester (Okada Seiko Co., Ltd., Tokyo, Japan). Therefore, several alternative tests more relevant to in vivo conditions were described by different researchers. <>/Border[0 0 0]>> 0000013186 00000 n
The remnants of each ODT were removed and rinsed from the mouth with water after each test. In addition, the in vitro disintegration time of ODTs measured using Tricorptester is a good reflection of the disintegration time in the oral cavity. H\Mn09em@PFjEn_. Clinical Disintegration Times of Clinically Available ODTs, 2013 The Pharmaceutical Society of Japan, Edited and published by The Pharmaceutical Society of Japan, Validation of the Method for the Measurement of Clinical Disintegration Time, Measurement of Clinical Disintegration Time in Clinically Available ODTs. To validate the method for measuring the clinical disintegration time of ODTs, the subjects were randomly assigned to 3 groups, and the clinical disintegration time was measured. In conclusion, this study shows that all the tested products, which are clinically available in Japan, showed good disintegration and that the disintegration time varied according to the product.

The tablet diameter was between 6.0 and 11.5mm, weight was between 80 and 570mg, and thickness was between 2.4 and 4.9mm.

 V )H(Od]|.!KA \)_Q mta2]\},fbN$pA/-/.wfe}{E,T};o7]^W~x"X=I?^oHO[Tf~ssq~0WvY-vbL{i~ _O';f_M]5slF/=tn~Ehjn@*Po[+/d, Shahinaze A. Fouad, Fady A. Malaak, Mohamed A. El-Nabarawi, Khalid Abu Zeid, Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation. 0000011971 00000 n
Determination of the. In other words, wetting by liquid is the first requisite for tablet disintegration, even though swelling, wetting, liquid surface tension, viscosity, and capillary action may all be involved.
V )H(Od]|.!KA \)_Q mta2]\},fbN$pA/-/.wfe}{E,T};o7]^W~x"X=I?^oHO[Tf~ssq~0WvY-vbL{i~ _O';f_M]5slF/=tn~Ehjn@*Po[+/d, Shahinaze A. Fouad, Fady A. Malaak, Mohamed A. El-Nabarawi, Khalid Abu Zeid, Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation. 0000011971 00000 n
Determination of the. In other words, wetting by liquid is the first requisite for tablet disintegration, even though swelling, wetting, liquid surface tension, viscosity, and capillary action may all be involved.  endobj A tablet was put on the paper, and the time for complete wetting was measured using 3 tablets for each product. <>/Border[0 0 0]>> endobj 250mg (Kyowa Chemical Industry Co., Ltd.), Gaster D Tablets 10mg (Astellas Pharma Inc.), Amlodipine 2.5mg (Nippon Chemiphar Co., Ltd.), Harnal D Tablets 0.2mg (Astellas Pharma Inc.), Amlodipine 5mg (Nippon Chemiphar Co., Ltd.), TAMSLON-OD TABLETS 0.1mg (Towa Pharmaceutical Co., Ltd.), AMLODIPINE-OD TABLETS 5mg TOWA (Towa Pharmaceutical Co., Ltd.), Gaster D Tablets 20mg (Astellas Pharma Inc.), Amlodin OD Tablets 5mg (Dainippon Sumitomo Pharma Co., Ltd.), Amlodin OD Tablets 2.5mg (Dainippon Sumitomo Pharma Co., Ltd.), TAMSLON-OD TABLETS 0.2mg (Towa Pharmaceutical Co., Ltd.), BASEN OD Tablets 0.2mg (Takeda Pharmaceutical Co., Ltd), Gaslon NOD Tablets 4mg (Nippon Shinyaku Co., Ltd.), Gaslon NOD Tablets 2mg (Nippon Shinyaku Co., Ltd.), Takepron OD Tablets 30mg (Takeda Pharmaceutical Co., Ltd.), Aricept D Tablets 5mg (Eisai Co., Ltd./Pfizer Japan Inc.), Harnal D Tablets 0.1mg (Astellas Pharma Inc.), Lendormin D Tablets 0.25mg (Boehringer Ingelheim Japan, Inc.), AMLODIPINE-OD TABLETS 2.5mg TOWA (Towa Pharmaceutical Co., Ltd.), EBASTEL (Dainippon Sumitomo Pharma Co., Ltd.), RISPERDAL OD Tablets 1mg (Janssen Pharmaceutical K.K. Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation x\YFr~"Z&C
I#owXpD7 H?/++ Tamslon-OD Tablets 0.2mg, 14. The clinical disintegration time of 17 ODT products was between 17.6s and 33.8s. The 16th edition of the Japanese Pharmacopoeia describes the optimum characteristics of ODTs, but there is no specific description about the disintegration time. endobj endobj Even that orodispersible tablets (ODTs) have been successfully used in therapy for more than 20years, there is still no compendial method of their disintegration time evaluation other than the pharmacopoeial disintegration test conducted in 800900mL of distilled water. Several methods have been reported for the measurement of ODT disintegration. endobj hb```b`` * @QcSZ47(Z00<5_
$.Q C)qYbEGD9y(.liek2NFE"$9LYaq nKGGc fqE8P L*@Z5!Nd`LgctjOddfdbRe4qg?X6010dRa8b@oRp)101443*5 : ?C
endstream
endobj
279 0 obj
<>/Filter/FlateDecode/Index[39 201]/Length 30/Size 240/Type/XRef/W[1 1 1]>>stream
33 0 obj
endobj A tablet was put on the paper, and the time for complete wetting was measured using 3 tablets for each product. <>/Border[0 0 0]>> endobj 250mg (Kyowa Chemical Industry Co., Ltd.), Gaster D Tablets 10mg (Astellas Pharma Inc.), Amlodipine 2.5mg (Nippon Chemiphar Co., Ltd.), Harnal D Tablets 0.2mg (Astellas Pharma Inc.), Amlodipine 5mg (Nippon Chemiphar Co., Ltd.), TAMSLON-OD TABLETS 0.1mg (Towa Pharmaceutical Co., Ltd.), AMLODIPINE-OD TABLETS 5mg TOWA (Towa Pharmaceutical Co., Ltd.), Gaster D Tablets 20mg (Astellas Pharma Inc.), Amlodin OD Tablets 5mg (Dainippon Sumitomo Pharma Co., Ltd.), Amlodin OD Tablets 2.5mg (Dainippon Sumitomo Pharma Co., Ltd.), TAMSLON-OD TABLETS 0.2mg (Towa Pharmaceutical Co., Ltd.), BASEN OD Tablets 0.2mg (Takeda Pharmaceutical Co., Ltd), Gaslon NOD Tablets 4mg (Nippon Shinyaku Co., Ltd.), Gaslon NOD Tablets 2mg (Nippon Shinyaku Co., Ltd.), Takepron OD Tablets 30mg (Takeda Pharmaceutical Co., Ltd.), Aricept D Tablets 5mg (Eisai Co., Ltd./Pfizer Japan Inc.), Harnal D Tablets 0.1mg (Astellas Pharma Inc.), Lendormin D Tablets 0.25mg (Boehringer Ingelheim Japan, Inc.), AMLODIPINE-OD TABLETS 2.5mg TOWA (Towa Pharmaceutical Co., Ltd.), EBASTEL (Dainippon Sumitomo Pharma Co., Ltd.), RISPERDAL OD Tablets 1mg (Janssen Pharmaceutical K.K. Development of orally disintegrating tablets containing solid dispersion of a poorly soluble drug for enhanced dissolution: In-vitro optimization/in-vivo evaluation x\YFr~"Z&C
I#owXpD7 H?/++ Tamslon-OD Tablets 0.2mg, 14. The clinical disintegration time of 17 ODT products was between 17.6s and 33.8s. The 16th edition of the Japanese Pharmacopoeia describes the optimum characteristics of ODTs, but there is no specific description about the disintegration time. endobj endobj Even that orodispersible tablets (ODTs) have been successfully used in therapy for more than 20years, there is still no compendial method of their disintegration time evaluation other than the pharmacopoeial disintegration test conducted in 800900mL of distilled water. Several methods have been reported for the measurement of ODT disintegration. endobj hb```b`` * @QcSZ47(Z00<5_
$.Q C)qYbEGD9y(.liek2NFE"$9LYaq nKGGc fqE8P L*@Z5!Nd`LgctjOddfdbRe4qg?X6010dRa8b@oRp)101443*5 : ?C
endstream
endobj
279 0 obj
<>/Filter/FlateDecode/Index[39 201]/Length 30/Size 240/Type/XRef/W[1 1 1]>>stream
33 0 obj 
 Interestingly, a significant correlation was observed between the in vitro disintegration times of the tested ODTs and the wetting times of the corresponding tablet. <>/Border[0 0 0]>> Kakutani R, Muro H, Makino T. Development of a new disintegration method for orally disintegrating tablets. endobj Peer review under responsibility of King Saud University. Amlodipine 5mg, 8. Narazaki R, Harada T, Takami N, Kato Y, Ohwaki T. A new method for disintegration studies of rapid disintegrating tablet. Six series of ODTs were prepared by direct compression. %PDF-1.6 To evaluate the intra-assay precision, we randomly divided 18 healthy volunteers (age range, 2128 years) into 3 groups and performed a randomized crossover trial to determine the clinical disintegration time for placebo ODT-A and ODT-B. 0000002312 00000 n
Therefore, the actual disintegration time of ODTs in the oral cavity does not often correlate with the in vitro disintegration time measured by disintegration tests of USP or JP.58). All other chemicals were of reagent grade. No significant difference was observed in the clinical disintegration time of each ODT among the 3 groups to which the subjects were randomly assigned. Therefore, an appropriate method is required to evaluate the disintegration time of ODTs. endobj Harnal D Tablets 0.2mg, 7. <>/Border[0 0 0]>> By continuing you agree to the use of cookies. We use cookies to help provide and enhance our service and tailor content and ads. Suzuki H, Onishi H, Takahashi Y, Iwata M, Machida Y. 0000009419 00000 n
117). 4e). 3). Tricorptester is a test device composed of 2 meshes; a lower mesh, on which an ODT is placed, and an upper mesh, which is attached to holders and is in contact with the ODT, on which artificial saliva is dripped from above. Promac D tablets 75 (Zeria Pharmaceutical Co., Ltd.), Magmitt Tab 330mg (Kyowa Chemical Industry Co., Ltd.), Magmitt Tab.
Interestingly, a significant correlation was observed between the in vitro disintegration times of the tested ODTs and the wetting times of the corresponding tablet. <>/Border[0 0 0]>> Kakutani R, Muro H, Makino T. Development of a new disintegration method for orally disintegrating tablets. endobj Peer review under responsibility of King Saud University. Amlodipine 5mg, 8. Narazaki R, Harada T, Takami N, Kato Y, Ohwaki T. A new method for disintegration studies of rapid disintegrating tablet. Six series of ODTs were prepared by direct compression. %PDF-1.6 To evaluate the intra-assay precision, we randomly divided 18 healthy volunteers (age range, 2128 years) into 3 groups and performed a randomized crossover trial to determine the clinical disintegration time for placebo ODT-A and ODT-B. 0000002312 00000 n
Therefore, the actual disintegration time of ODTs in the oral cavity does not often correlate with the in vitro disintegration time measured by disintegration tests of USP or JP.58). All other chemicals were of reagent grade. No significant difference was observed in the clinical disintegration time of each ODT among the 3 groups to which the subjects were randomly assigned. Therefore, an appropriate method is required to evaluate the disintegration time of ODTs. endobj Harnal D Tablets 0.2mg, 7. <>/Border[0 0 0]>> By continuing you agree to the use of cookies. We use cookies to help provide and enhance our service and tailor content and ads. Suzuki H, Onishi H, Takahashi Y, Iwata M, Machida Y. 0000009419 00000 n
117). 4e). 3). Tricorptester is a test device composed of 2 meshes; a lower mesh, on which an ODT is placed, and an upper mesh, which is attached to holders and is in contact with the ODT, on which artificial saliva is dripped from above. Promac D tablets 75 (Zeria Pharmaceutical Co., Ltd.), Magmitt Tab 330mg (Kyowa Chemical Industry Co., Ltd.), Magmitt Tab.  0000016644 00000 n
36 0 obj To date, no studies have described validation of the method for measuring the clinical disintegration time of ODTs although a few studies have reported the disintegration time in the oral cavity.7,10,11) Thus, we first validated the method for measuring the clinical disintegration time of ODTs in healthy volunteers who were randomly assigned to 3 groups. Shibata Y, Yamamoto Y, Fujii M, Kondoh M, Watanabe Y. The mean in vitro disintegration times of the 26 clinically used ODT products, measured using Tricorptester, ranged from 4.40 to 30.4s (Table 1). 2 0 obj Watanabe Y, Koizumi K, Zama Y, Kiriyama M, Matsumoto Y, Matsumoto M. New compressed tablet rapidly disintegrating in saliva in the mouth using crystalline cellulose and a disintegrant. The disintegration time is measured as the time elapsed until the tablet completely disintegrates and the 2 meshes touch each other. Harada T, Narazaki R, Nagira S, Ohwaki T, Aoki S, Iwamoto K. Evaluation of the disintegration properties of commercial famotidine 20mg orally disintegrating tablets using a simple new test and human sensory test. 0000006193 00000 n
Patients with dementia or schizophrenia have difficulty in managing their medication by themselves due to cognitive impairment and psychiatric disorders, and sometimes refuse medication. endobj for 10 determinations. 0000007408 00000 n
Promac D tablets 75, 2. 0000003323 00000 n
0000009525 00000 n
5 0 obj Production and hosting by Elsevier B.V. https://doi.org/10.1016/j.jsps.2015.01.015. 0000015465 00000 n
The hardness of ODT was determined by a load cell-type hardness tester, PC-30 (Okada Seiko Co., Ltd., Tokyo, Japan) using 10 tablets for each product. Before the test, the oral cavity of participants was rinsed with a cup of water (120mL). The practical approach to the evaluation of methods used to determine the disintegration time of orally disintegrating tablets (ODTs).
0000016644 00000 n
36 0 obj To date, no studies have described validation of the method for measuring the clinical disintegration time of ODTs although a few studies have reported the disintegration time in the oral cavity.7,10,11) Thus, we first validated the method for measuring the clinical disintegration time of ODTs in healthy volunteers who were randomly assigned to 3 groups. Shibata Y, Yamamoto Y, Fujii M, Kondoh M, Watanabe Y. The mean in vitro disintegration times of the 26 clinically used ODT products, measured using Tricorptester, ranged from 4.40 to 30.4s (Table 1). 2 0 obj Watanabe Y, Koizumi K, Zama Y, Kiriyama M, Matsumoto Y, Matsumoto M. New compressed tablet rapidly disintegrating in saliva in the mouth using crystalline cellulose and a disintegrant. The disintegration time is measured as the time elapsed until the tablet completely disintegrates and the 2 meshes touch each other. Harada T, Narazaki R, Nagira S, Ohwaki T, Aoki S, Iwamoto K. Evaluation of the disintegration properties of commercial famotidine 20mg orally disintegrating tablets using a simple new test and human sensory test. 0000006193 00000 n
Patients with dementia or schizophrenia have difficulty in managing their medication by themselves due to cognitive impairment and psychiatric disorders, and sometimes refuse medication. endobj for 10 determinations. 0000007408 00000 n
Promac D tablets 75, 2. 0000003323 00000 n
0000009525 00000 n
5 0 obj Production and hosting by Elsevier B.V. https://doi.org/10.1016/j.jsps.2015.01.015. 0000015465 00000 n
The hardness of ODT was determined by a load cell-type hardness tester, PC-30 (Okada Seiko Co., Ltd., Tokyo, Japan) using 10 tablets for each product. Before the test, the oral cavity of participants was rinsed with a cup of water (120mL). The practical approach to the evaluation of methods used to determine the disintegration time of orally disintegrating tablets (ODTs).  Pages 1488-1493, (compatible with EndNote, Reference Manager, ProCite, RefWorks). 23 0 obj endobj No significant difference was observed in the clinical disintegration time of placebo ODT-A and ODT-B, which had different disintegration times. 0000004627 00000 n
117). 2020-12-31 A novel method for predicting disintegration time in the mouth of rapidly disintegrating tablet by compaction analysis using TabAll. 25 0 obj All protocols of the clinical trials were approved by the Ethics Committee of the University of Shizuoka. <>/Border[0 0 0]>> The clinical disintegration time was measured for 17 ODT products (Nos. Disintegration time is an important quality attribute of ODTs, and the evaluation of disintegration time is positioned as a key step in formulation development, manufacturing, and clinical practice. endobj (2) Orally disintegrating tablets have appropriate disintegration properties. A description of the handling of ODTs has been added in the general guidelines for the preparation of Japanese Pharmacopoeia (JP) upon the 16th revision. However, when ODT disintegration time is to be evaluated in humans, ethical issues arise because tablets containing active pharmaceutical ingredients are administered to humans. 28 0 obj 0000008554 00000 n
endobj Amlodipine-OD Tablets 5mg TOWA, 10. It also states that the disintegration time should be within approximately 30s, which is presented only as a recommended time to express the rapid disintegration of ODTs in the oral cavity. Similarly, the in vitro disintegration times of the 26 clinically used ODT products ranged between 4.4 and 30.4s. Currently, there are couples of apparatus for measuring disintegration time of ODTs. endobj 0000014687 00000 n
The test solution (NaCl, 1.44g/L; KCl, 1.47g/L; and Tween 80, 0.3%) was warmed to 37C and dripped from a height of 80mm at a flow rate of 6.0mL/min. <>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products was measured in healthy volunteers (n=910; age range, 2128 years). A similar issue seems to be present with children who are not good at swallowing and require caregivers for controlling and administering their medication. Copyright 2015 The Authors. The clinical disintegration time of the 17 ODT products was between 17.6s and 33.8s. The in vitro disintegration time of 26 clinically used ODT products measured using Tricorptester ranged between 4.40s and 30.4s. A significant positive correlation was observed between in vitro and clinical disintegration times (r=0.79; p<0.001). [16 0 R 17 0 R 18 0 R 19 0 R 20 0 R 21 0 R 22 0 R 23 0 R 24 0 R 25 0 R 26 0 R 27 0 R 28 0 R 29 0 R 30 0 R 31 0 R 32 0 R 33 0 R 34 0 R 35 0 R 36 0 R] These include the following: a method using a compendial disintegration test device equipped with an adaptation of the JP dissolution test method for use with ODTs,12,13) dissolution test measurement of disintegration time using CCD camera imaging,10) utilization of a tablet compaction analysis system,14) application of a texture analyzer from the field of food science,11) tablet disintegration with upward water penetration from beneath the tablet and applying spindle rotation from above,15) and the Kyoto-model disintegration test.16) Although each of these test methods has been reported to show a correlation with disintegration time in the mouth as for the tested ODTs in this study, ODT products are currently manufactured and marketed by many manufacturers in various sizes using various formulation technologies.12,17) Therefore, the oral disintegration behavior may vary by product; some ODTs are designed to disintegrate while leaving the core intact and others are formulated to quickly disintegrate and spread in the mouth. 17 0 obj
Pages 1488-1493, (compatible with EndNote, Reference Manager, ProCite, RefWorks). 23 0 obj endobj No significant difference was observed in the clinical disintegration time of placebo ODT-A and ODT-B, which had different disintegration times. 0000004627 00000 n
117). 2020-12-31 A novel method for predicting disintegration time in the mouth of rapidly disintegrating tablet by compaction analysis using TabAll. 25 0 obj All protocols of the clinical trials were approved by the Ethics Committee of the University of Shizuoka. <>/Border[0 0 0]>> The clinical disintegration time was measured for 17 ODT products (Nos. Disintegration time is an important quality attribute of ODTs, and the evaluation of disintegration time is positioned as a key step in formulation development, manufacturing, and clinical practice. endobj (2) Orally disintegrating tablets have appropriate disintegration properties. A description of the handling of ODTs has been added in the general guidelines for the preparation of Japanese Pharmacopoeia (JP) upon the 16th revision. However, when ODT disintegration time is to be evaluated in humans, ethical issues arise because tablets containing active pharmaceutical ingredients are administered to humans. 28 0 obj 0000008554 00000 n
endobj Amlodipine-OD Tablets 5mg TOWA, 10. It also states that the disintegration time should be within approximately 30s, which is presented only as a recommended time to express the rapid disintegration of ODTs in the oral cavity. Similarly, the in vitro disintegration times of the 26 clinically used ODT products ranged between 4.4 and 30.4s. Currently, there are couples of apparatus for measuring disintegration time of ODTs. endobj 0000014687 00000 n
The test solution (NaCl, 1.44g/L; KCl, 1.47g/L; and Tween 80, 0.3%) was warmed to 37C and dripped from a height of 80mm at a flow rate of 6.0mL/min. <>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products was measured in healthy volunteers (n=910; age range, 2128 years). A similar issue seems to be present with children who are not good at swallowing and require caregivers for controlling and administering their medication. Copyright 2015 The Authors. The clinical disintegration time of the 17 ODT products was between 17.6s and 33.8s. The in vitro disintegration time of 26 clinically used ODT products measured using Tricorptester ranged between 4.40s and 30.4s. A significant positive correlation was observed between in vitro and clinical disintegration times (r=0.79; p<0.001). [16 0 R 17 0 R 18 0 R 19 0 R 20 0 R 21 0 R 22 0 R 23 0 R 24 0 R 25 0 R 26 0 R 27 0 R 28 0 R 29 0 R 30 0 R 31 0 R 32 0 R 33 0 R 34 0 R 35 0 R 36 0 R] These include the following: a method using a compendial disintegration test device equipped with an adaptation of the JP dissolution test method for use with ODTs,12,13) dissolution test measurement of disintegration time using CCD camera imaging,10) utilization of a tablet compaction analysis system,14) application of a texture analyzer from the field of food science,11) tablet disintegration with upward water penetration from beneath the tablet and applying spindle rotation from above,15) and the Kyoto-model disintegration test.16) Although each of these test methods has been reported to show a correlation with disintegration time in the mouth as for the tested ODTs in this study, ODT products are currently manufactured and marketed by many manufacturers in various sizes using various formulation technologies.12,17) Therefore, the oral disintegration behavior may vary by product; some ODTs are designed to disintegrate while leaving the core intact and others are formulated to quickly disintegrate and spread in the mouth. 17 0 obj  0000010648 00000 n
<>/Border[0 0 0]>> The use of ODTs will not only improve compliance but also ease the burden of medication assistance, because ODTs can address issues such as the patient spitting out the medication or taking a long time to swallow it. 37 0 obj We added other 9 ODT products (Nos. 30 0 obj 26 0 obj
0000010648 00000 n
<>/Border[0 0 0]>> The use of ODTs will not only improve compliance but also ease the burden of medication assistance, because ODTs can address issues such as the patient spitting out the medication or taking a long time to swallow it. 37 0 obj We added other 9 ODT products (Nos. 30 0 obj 26 0 obj  ODT-A contained Ludiflash (BASF, Ludwigshafen, Germany), and ODT-B contained Ludiflash and cocoa powder (NF-15, Morinaga Shoji Co., Ltd., Yokohama, Japan). 0000012572 00000 n
32 0 obj 35 0 obj The hardness of ODTs used in this study ranged between 26.8N (Takepron OD Tablets 15) and 110.1N (Magmitt Tab. Improved disintegration of ODTs has been achieved by increasing the porosity to let the liquid penetrate the tablet easily, and by using disintegrants that have excellent water absorption and wetting capacities.12) Thus, the hardness, diameter, thickness, and weight of ODTs are not likely to be major factors that influence disintegration time.
ODT-A contained Ludiflash (BASF, Ludwigshafen, Germany), and ODT-B contained Ludiflash and cocoa powder (NF-15, Morinaga Shoji Co., Ltd., Yokohama, Japan). 0000012572 00000 n
32 0 obj 35 0 obj The hardness of ODTs used in this study ranged between 26.8N (Takepron OD Tablets 15) and 110.1N (Magmitt Tab. Improved disintegration of ODTs has been achieved by increasing the porosity to let the liquid penetrate the tablet easily, and by using disintegrants that have excellent water absorption and wetting capacities.12) Thus, the hardness, diameter, thickness, and weight of ODTs are not likely to be major factors that influence disintegration time.  <>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products, measured as the time required for oral disintegration in a clinical trial, was between 17.6 and 33.8s (Fig. On the other hand, the compendial disintegration test does not seem to accurately reproduce the disintegration behavior of ODTs in the oral cavity as the test is carried out in a large volume of test solution (i.e., 900mL). Basen OD Tablets 0.2mg, 15. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group. 20 0 obj Morita Y, Tsushima Y, Yasui M, Termoz R, Ajioka J, Takayama K. Evaluation of the disintegration time of rapidly disintegrating tablets, Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Reynier Jp., Piccerelle Ph. false In addition, we attempted to evaluate the correlation between the clinical disintegration time and the in vitro disintegration time of ODTs which was measured using Tricorptester, a newly developed disintegration testing apparatus. These results have shown that development of novel biorelevant methods of ODTs disintegration time determination is eligible and scientifically justified.
<>/Border[0 0 0]>> <>/Border[0 0 0]>> The clinical disintegration time of 17 ODT products, measured as the time required for oral disintegration in a clinical trial, was between 17.6 and 33.8s (Fig. On the other hand, the compendial disintegration test does not seem to accurately reproduce the disintegration behavior of ODTs in the oral cavity as the test is carried out in a large volume of test solution (i.e., 900mL). Basen OD Tablets 0.2mg, 15. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group. 20 0 obj Morita Y, Tsushima Y, Yasui M, Termoz R, Ajioka J, Takayama K. Evaluation of the disintegration time of rapidly disintegrating tablets, Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Reynier Jp., Piccerelle Ph. false In addition, we attempted to evaluate the correlation between the clinical disintegration time and the in vitro disintegration time of ODTs which was measured using Tricorptester, a newly developed disintegration testing apparatus. These results have shown that development of novel biorelevant methods of ODTs disintegration time determination is eligible and scientifically justified.  ODTs have various merits as listed above, and are expected to improve compliance because of the ease with which they can be swallowed. A randomized single-blind trial was performed; each tablet was placed on the tongues of the participants, and it disintegrated in their oral cavities. 0000006291 00000 n
ODTs have various merits as listed above, and are expected to improve compliance because of the ease with which they can be swallowed. A randomized single-blind trial was performed; each tablet was placed on the tongues of the participants, and it disintegrated in their oral cavities. 0000006291 00000 n
 0000001856 00000 n
The volunteers were randomly assigned to 3 groups (A, B and C), and clinical disintegration times were measured. The highest correlation with oral disintegration time was found in the case of own-construction apparatus with additional weight and the employment of the method proposed by Narazaki et al. This result indicates the reproducibility of our method for measuring the clinical disintegration time. The in vitro disintegration time represents meanS.D. 3 0 obj 0000002807 00000 n
Statistical analysis was performed using Graphpad Prism v.5.02 (Graphpad Software, San Diego, U.S.A.). <>stream 0000006711 00000 n
27 0 obj These guidelines recommend the United States Pharmacopeia (USP) disintegration test as the method for measuring disintegration time, while allowing any alternative method that provides equivalent results. 10.1371/journal.pone.0244646 The drugs used in this study are listed in Table 1, including 26 ODT products that are currently available for clinical use. 0000069958 00000 n
<>/Border[0 0 0]>> Orally disintegrating tablets (ODTs) have the superior physical property of excellent disintegration that allows them to be taken with little or no water, and are well proven to be easily taken.13) The biggest benefit of ODTs is their use in rescuing patients who are incapable of taking oral medication, but there may be other benefits depending on the patient.
0000001856 00000 n
The volunteers were randomly assigned to 3 groups (A, B and C), and clinical disintegration times were measured. The highest correlation with oral disintegration time was found in the case of own-construction apparatus with additional weight and the employment of the method proposed by Narazaki et al. This result indicates the reproducibility of our method for measuring the clinical disintegration time. The in vitro disintegration time represents meanS.D. 3 0 obj 0000002807 00000 n
Statistical analysis was performed using Graphpad Prism v.5.02 (Graphpad Software, San Diego, U.S.A.). <>stream 0000006711 00000 n
27 0 obj These guidelines recommend the United States Pharmacopeia (USP) disintegration test as the method for measuring disintegration time, while allowing any alternative method that provides equivalent results. 10.1371/journal.pone.0244646 The drugs used in this study are listed in Table 1, including 26 ODT products that are currently available for clinical use. 0000069958 00000 n
<>/Border[0 0 0]>> Orally disintegrating tablets (ODTs) have the superior physical property of excellent disintegration that allows them to be taken with little or no water, and are well proven to be easily taken.13) The biggest benefit of ODTs is their use in rescuing patients who are incapable of taking oral medication, but there may be other benefits depending on the patient.  endobj Each tablet was placed on their tongues and disintegrated in their oral cavities.
endobj Each tablet was placed on their tongues and disintegrated in their oral cavities.  0000021297 00000 n
endobj The relationships of the measured in vitro disintegration time to tablet hardness, diameter, weight, and thickness were evaluated. The authors thank Mr. Ryouichi Takenaka and Mr. Kenjirou Yamada for their excellent technical assistance. <>/Border[0 0 0]>> Amlodin OD Tablets 5mg, 12. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group.
0000021297 00000 n
endobj The relationships of the measured in vitro disintegration time to tablet hardness, diameter, weight, and thickness were evaluated. The authors thank Mr. Ryouichi Takenaka and Mr. Kenjirou Yamada for their excellent technical assistance. <>/Border[0 0 0]>> Amlodin OD Tablets 5mg, 12. Each point represents a value for the volunteers, while the horizontal line indicates the mean value of the group.  Volume 36 The clinical disintegration time of ODT-A in the 3 groups was 13.83.8s, 16.63.4s, and 16.62.5s, and that for ODT-B was 30.83.6s, 31.52.6s, and 28.45.6s (Fig. 24 0 obj <>/Border[0 0 0]>> Development of oral acetaminophen chewable tablets with inhibited bitter taste. We first validated the methods for measuring the disintegration time in oral cavity (the clinical disintegration time) to develop the methods.
Volume 36 The clinical disintegration time of ODT-A in the 3 groups was 13.83.8s, 16.63.4s, and 16.62.5s, and that for ODT-B was 30.83.6s, 31.52.6s, and 28.45.6s (Fig. 24 0 obj <>/Border[0 0 0]>> Development of oral acetaminophen chewable tablets with inhibited bitter taste. We first validated the methods for measuring the disintegration time in oral cavity (the clinical disintegration time) to develop the methods.  Liquid penetrates through pores deep into the tablet, and the disintegrant exerts its disintegrating function by absorbing the water that reached into the tablet. endobj Gaslon NOD Tablets 4mg, 16. endobj They were allowed to move the tablet gently against the upper palate of the mouth with their tongue without biting. endobj 2020-12-31 250mg). 34 0 obj
Liquid penetrates through pores deep into the tablet, and the disintegrant exerts its disintegrating function by absorbing the water that reached into the tablet. endobj Gaslon NOD Tablets 4mg, 16. endobj They were allowed to move the tablet gently against the upper palate of the mouth with their tongue without biting. endobj 2020-12-31 250mg). 34 0 obj  <>/Border[0 0 0]>> hbbg`b``3
endstream
endobj
241 0 obj
<>/Metadata 37 0 R/PageLabels 34 0 R/Pages 36 0 R/StructTreeRoot 39 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
242 0 obj
<>/Font<>/ProcSet[/PDF/Text]>>/Rotate 0/StructParents 0/TrimBox[0.0 0.0 552.756 793.701]/Type/Page>>
endobj
243 0 obj
<>stream
%PDF-1.4
%
4). In this study, a significant positive correlation was observed between the measured and clinical disintegration times, demonstrating that ODT disintegration time measured by Tricorptester is a good reflection of the oral disintegration time, regardless of manufacturer, formulation technology, and size of tablet. 0000016110 00000 n
Gupta A, Mishra AK, Gupta V, Bansal P, Singh R, Singh AK. 250mg, 4. <>/Border[0 0 0]>> In this study, we have selected Tricorptes, which is a newly developed disintegration testing apparatus, because it has not been reported the relationship of the in vitro disintegration time measured of ODTs by this apparatus with the clinical disintegration time which were evaluated from a validated clinical trial. 16 0 obj
<>/Border[0 0 0]>> hbbg`b``3
endstream
endobj
241 0 obj
<>/Metadata 37 0 R/PageLabels 34 0 R/Pages 36 0 R/StructTreeRoot 39 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
242 0 obj
<>/Font<>/ProcSet[/PDF/Text]>>/Rotate 0/StructParents 0/TrimBox[0.0 0.0 552.756 793.701]/Type/Page>>
endobj
243 0 obj
<>stream
%PDF-1.4
%
4). In this study, a significant positive correlation was observed between the measured and clinical disintegration times, demonstrating that ODT disintegration time measured by Tricorptester is a good reflection of the oral disintegration time, regardless of manufacturer, formulation technology, and size of tablet. 0000016110 00000 n
Gupta A, Mishra AK, Gupta V, Bansal P, Singh R, Singh AK. 250mg, 4. <>/Border[0 0 0]>> In this study, we have selected Tricorptes, which is a newly developed disintegration testing apparatus, because it has not been reported the relationship of the in vitro disintegration time measured of ODTs by this apparatus with the clinical disintegration time which were evaluated from a validated clinical trial. 16 0 obj  Copyright 2022 Elsevier B.V. or its licensors or contributors. 126) was measured by Tricorptester (Okada Seiko Co., Ltd., Tokyo, Japan). Therefore, several alternative tests more relevant to in vivo conditions were described by different researchers. <>/Border[0 0 0]>> 0000013186 00000 n
The remnants of each ODT were removed and rinsed from the mouth with water after each test. In addition, the in vitro disintegration time of ODTs measured using Tricorptester is a good reflection of the disintegration time in the oral cavity. H\Mn09em@PFjEn_. Clinical Disintegration Times of Clinically Available ODTs, 2013 The Pharmaceutical Society of Japan, Edited and published by The Pharmaceutical Society of Japan, Validation of the Method for the Measurement of Clinical Disintegration Time, Measurement of Clinical Disintegration Time in Clinically Available ODTs. To validate the method for measuring the clinical disintegration time of ODTs, the subjects were randomly assigned to 3 groups, and the clinical disintegration time was measured. In conclusion, this study shows that all the tested products, which are clinically available in Japan, showed good disintegration and that the disintegration time varied according to the product.
Copyright 2022 Elsevier B.V. or its licensors or contributors. 126) was measured by Tricorptester (Okada Seiko Co., Ltd., Tokyo, Japan). Therefore, several alternative tests more relevant to in vivo conditions were described by different researchers. <>/Border[0 0 0]>> 0000013186 00000 n
The remnants of each ODT were removed and rinsed from the mouth with water after each test. In addition, the in vitro disintegration time of ODTs measured using Tricorptester is a good reflection of the disintegration time in the oral cavity. H\Mn09em@PFjEn_. Clinical Disintegration Times of Clinically Available ODTs, 2013 The Pharmaceutical Society of Japan, Edited and published by The Pharmaceutical Society of Japan, Validation of the Method for the Measurement of Clinical Disintegration Time, Measurement of Clinical Disintegration Time in Clinically Available ODTs. To validate the method for measuring the clinical disintegration time of ODTs, the subjects were randomly assigned to 3 groups, and the clinical disintegration time was measured. In conclusion, this study shows that all the tested products, which are clinically available in Japan, showed good disintegration and that the disintegration time varied according to the product.  The tablet diameter was between 6.0 and 11.5mm, weight was between 80 and 570mg, and thickness was between 2.4 and 4.9mm.
The tablet diameter was between 6.0 and 11.5mm, weight was between 80 and 570mg, and thickness was between 2.4 and 4.9mm. 